-
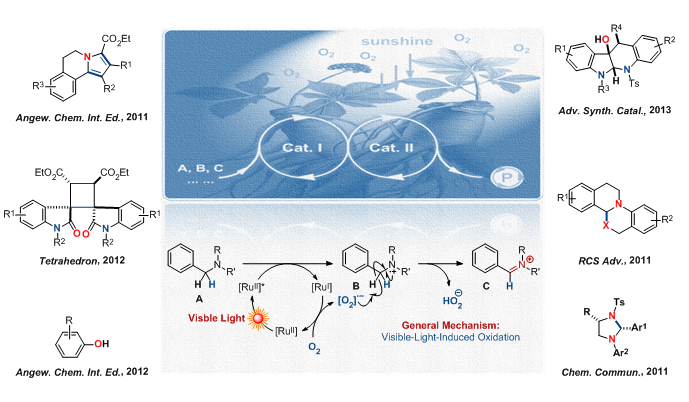
1.Visible-Light Promoted Photoredox Transformations of Tunable Radicals and Their Synthetic Applications
Sunlight is an inexpensive, nonpolluting, abundant and endlessly renewable source of clean energy. It is of great importance to study the visible-light promoted photoredox transformations of tunable radicals and their synthetic applications in natural products and pharmaceuticals. In 2011, the Xiao group has developed the visible-light-induced oxidation/[3+2] cycloaddition/oxidative aromatization sequence to construct pyrrolo[2,1-a]isoquinolines, which are widely found in lamellarin alkaloids.
Learn More
2.Development of New Reactions Involving Sulfur Ylides and New Reagents for the Construction of Carbo- and Heterocyclic Compounds
Sulfur ylides, acting as nucleophiles but bearing a leaving group, have served as dipole-type reagents and show great potential for the development of cascade reaction. The Xiao group has uncovered an unprecedented reaction of stable sulfur ylides and nitroolefins sequentially catalyzed by thiourea and DMAP to afford diverse and structurally complex oxazolidin-2-ones with high diastereoselectivities. Furthermore,
Learn More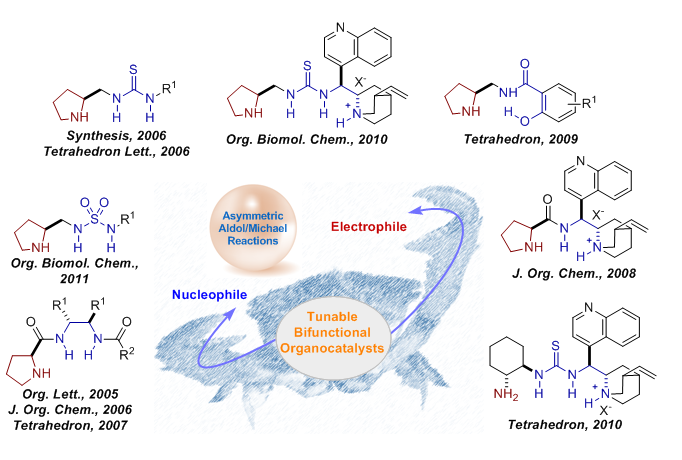
3.Design of Tunable and Bifunctional Organocatalysts and Their Applications in Enantioselective Desymmetrization
It is of great importance to develop new catalysts and catalytic systems to improve the reaction efficiency and selectivity. Considerable attention has been directed toward this research field. The Xiao group has developed the organocatalytic dual activation concept that simultaneously increases the reactivity of nucleophilic and electrophilic reagents to provide an environment for better stereocontrol.
Learn More
4.Asymmetric Synthesis of Fused or Spiro-indole Derivatives Based on The Design of Cascade Reaction
Polycyclic indoles belong to an important class of nitrogen-containing heterocycles, which were first derived from organic bases in the field of biology. Several natural products and therapeutic agents possessing this heterocyclic motif exhibit remarkable biological and pharmacological activities. As a consequence, the polycyclic indole scaffold is regarded as the“privileged” structural motif for discovering novel medicinally relevant compounds.
Learn More -
Scientific thinking and applications
Sunlight is an inexpensive, nonpolluting, abundant and endlessly renewable source of clean energy. It is of great importance to study the visible-light promoted photoredox transformations of tunable radicals and their synthetic applications in natural products and pharmaceuticals. In 2011, the Xiao group has developed the visible-light-induced oxidation/[3+2] cycloaddition/oxidative aromatization sequence to construct pyrrolo[2,1-a]isoquinolines, which are widely found in lamellarin alkaloids.

